A key question in neurobiology is how pathology is initiated and propagated in the brain of patients with neurodegenerative disorders. A major focus of studies in Dr. Efrat Levy’s laboratory has been the pathogenic factors that contribute to the development of Alzheimer’s disease (AD), using a multidisciplinary approach to identify factors that initiate, promote, or inhibit the disease. To this end the laboratory has been employing molecular, biochemical, cellular, and transgenic mouse methods.
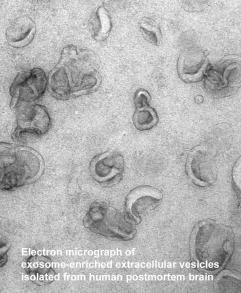
Dr. Levy has initiated novel directions in the research of amyloidosis in general and AD in particular. In recent years she has been studying vesicular trafficking, and the laboratory’s studies of vesicular transport laid the groundwork for developing the models and methods to study the role of a novel bioactive vesicle, the exosome, as either a protective or pathogenic vehicle in the brain.
The laboratory has been pursuing the hypothesis that exosome secretion is a mechanism for clearance of endocytic vesicular content when dysfunctions in the endosomal-lysosomal pathway prevent the transport of cargo for degradation in the lysosomes.
Paradoxically, the elimination of cytotoxic material from the cell via exosomes may cause spread of toxic material in the central nervous system, promoting the development of neurodegenerative diseases, such as AD.
The laboratory has found that human and mouse brain exosomes are enriched with the carboxyl-terminal fragments of the amyloid beta precursor protein, neurotoxic proteins that are a source of amyloid beta (Abeta), the major constituent of the amyloid deposited in the brain of AD patients. Thus, exosomes are potentially capable of generating Abeta at sites distant from the cell that secreted the exosomes. Additionally, Abeta binds to the surface of the lipid-rich exosomal membrane, potentially forming a seeding site for amyloid.
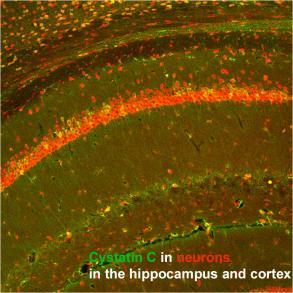
Studies are underway in the laboratory to investigate the pathogenic function of brain exosomes. In parallel, research is conducted of the neuroprotection activities of cystatin C (CysC).
The laboratory had shown that CysC alleviates endosomal-lysosomal abnormalities and memory deficits in mouse models of Down syndrome and of progressive myoclonus epilepsy, and preliminary studies have recently shown that CysC enhances exosome secretion – suggesting a mechanism by which CysC can be neuroprotective in neurodegenerative disorders with endosomal and/or exosomal disruption.